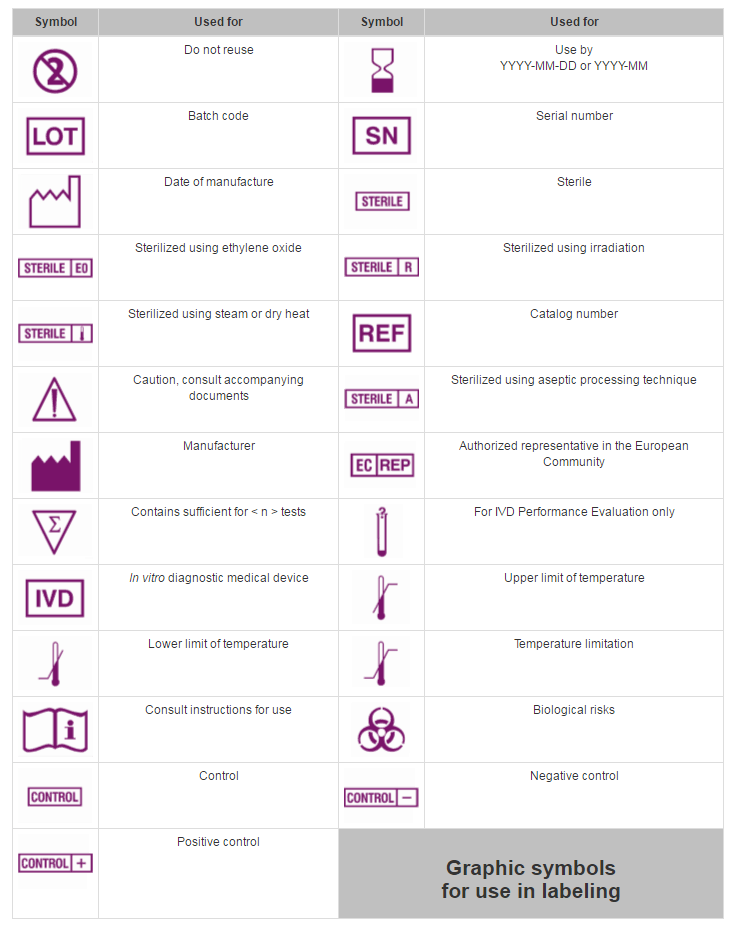
Medical Device Label Font Size . The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Guidance documents for medical devices. Here is the list of guidance documents with relevant forms and. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro.
from www.lexology.com
Guidance documents for medical devices. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Here is the list of guidance documents with relevant forms and. Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009.
FDA Issues Final Rule on Use of Symbols in Labeling Lexology
Medical Device Label Font Size Here is the list of guidance documents with relevant forms and. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Guidance documents for medical devices. Here is the list of guidance documents with relevant forms and. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Label must have indication if the device incorporates:
From www.st-fda.com
FDA认证药品标签和成分要求 Medical Device Label Font Size The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Label must have indication if the device incorporates: Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Here is the list of guidance documents with relevant forms. Medical Device Label Font Size.
From www.fontineed.com
Medicals Font, Download Medicals .ttf truetype or .zip Free FontIneed Medical Device Label Font Size The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Here is the list of guidance documents with relevant forms and. Guidance documents for medical devices. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Label must have indication if. Medical Device Label Font Size.
From www.freseniusmedicalcare.com
Medical Device Regulation Fresenius Medical Care Medical Device Label Font Size Label must have indication if the device incorporates: Here is the list of guidance documents with relevant forms and. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Overall, the. Medical Device Label Font Size.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Label Font Size Label must have indication if the device incorporates: Here is the list of guidance documents with relevant forms and. Guidance documents for medical devices. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Medical Device Label Font Size.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Label Font Size Here is the list of guidance documents with relevant forms and. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Guidance documents for medical devices. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. The commission’s. Medical Device Label Font Size.
From ectcolllefi.cf
Iso 15223 1 2012 Medical Devices symbols to be Used with Medical device Medical Device Label Font Size Guidance documents for medical devices. Here is the list of guidance documents with relevant forms and. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Label must have indication if the device incorporates: The commission’s guideline on the readability of the label and package leaflet of. Medical Device Label Font Size.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Medical Device Label Font Size The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Guidance documents for medical devices. Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Overall, the agency recommends that the font size. Medical Device Label Font Size.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Label Font Size The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Label must have indication if the device incorporates: Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Here is the list of guidance documents with relevant forms. Medical Device Label Font Size.
From mungfali.com
Medical Device Labeling Symbols Medical Device Label Font Size Here is the list of guidance documents with relevant forms and. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Label must have indication if the device incorporates: Guidance documents for medical devices. The purpose of this imdrf guidance is to provide globally harmonized labelling principles. Medical Device Label Font Size.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Label Font Size The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Here is the list of guidance documents with relevant forms and. Guidance documents for medical devices. Label must have indication if. Medical Device Label Font Size.
From www.pinterest.ph
medical fonts Best Fonts Medical font, Cool fonts, Best Medical Device Label Font Size Guidance documents for medical devices. Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Here is the list of. Medical Device Label Font Size.
From 54.84.202.198
How To Design Effective Medical Logo DesignMantic The Design Shop Medical Device Label Font Size Guidance documents for medical devices. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Label must have indication if the device incorporates: Here is the list of guidance documents with. Medical Device Label Font Size.
From templates.rjuuc.edu.np
Medical Device Label Template Medical Device Label Font Size Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Guidance documents for medical devices. Here is the list of. Medical Device Label Font Size.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Font Size The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Here is the list of guidance documents with relevant forms and. Label must have indication if the device incorporates: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Overall, the. Medical Device Label Font Size.
From mavink.com
Medical Device Labeling Symbols Medical Device Label Font Size The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Here is the list of guidance documents with relevant forms and. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Guidance documents for medical devices. The commission’s. Medical Device Label Font Size.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Label Font Size The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Here is the list of guidance documents with relevant forms and. Label must have indication if the device incorporates: Guidance documents. Medical Device Label Font Size.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Medical Device Label Font Size Here is the list of guidance documents with relevant forms and. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro. Guidance documents for medical devices. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. The commission’s. Medical Device Label Font Size.
From wqs.de
Kennzeichnung Bildzeichen Medizinprodukte WQS Medical Device Label Font Size The commission’s guideline on the readability of the label and package leaflet of medicinal products for human use from 2009. Guidance documents for medical devices. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Label must have indication if the device incorporates: Here is the list. Medical Device Label Font Size.